Periodic Properties of the Elements
Electronic Affinities
Atoms can also gain electrons to form negatively charged ions (anions)
The electron affinity is the energy change associated with an atom or ion in the gas state gaining an electron.
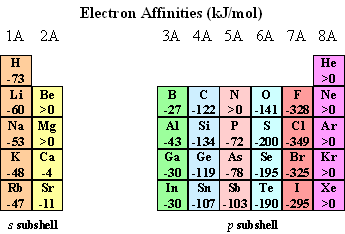
Cl(g) + e- -> Cl-(g) DE = -328 kJ/mol
Thus, we say that chlorine has an electron affinity of -328 kJ/mol.
The greater the attraction for the electron, the more exothermic the process
For anions and some neutral atoms, added an electron is an endothermic process, i.e. work must be done to force an electron onto the atom. This results in the formation of an unstable anion.

The general trend is for the electron affinity to become increasingly negative (stronger binding of an electron) as we move across each period toward the halogens.
Electron affinities do not change much as we move down a group
|
Element |
Ion |
E (kJ/mol) |
|
F |
F- |
-328 |
|
Cl |
Cl- |
-349 |
|
Br |
Br- |
-325 |
|
I |
I- |
-295 |
1996 Michael Blaber